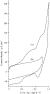APPENDIX
II-BJ: Searching for an Inexpensive Test
for WNV 1: Ionescu,
et al, “Amperometric Immunosensor for the
Detection of Anti-West Nile Virus IgG Using a
Photoactive Copolymer,” Enzyme and Macrobial Technology,
volume 40, issue 3, 5 February 2007, pages 403-408.
This appendix is copied
from:
linkinghub.elsevier.com/retrieve/pii/S0141022906003619
Click on:
doi:10.1016/j.enzmictec.2006.07.010
|
|
|
|
Enzyme and Microbial Technology |
|
|
|
doi:10.1016/j.enzmictec.2006.07.010 ![]()
Copyright ©
2006 Elsevier Inc. All rights reserved.
Amperometric immunosensor for the detection of anti-West
Rodica E. Ionescua, Serge Cosniera, ![]() ,
, ![]() , Gregoire
Herzoga, Karine
Gorgya, Boaz Leshemb, Sebastien
Herrmannb and Robert S. Marksb, c, d
, Gregoire
Herzoga, Karine
Gorgya, Boaz Leshemb, Sebastien
Herrmannb and Robert S. Marksb, c, d
aLaboratoire d’Electrochimie Organique et de Photochimie Redox, UMR CNRS 5630,
Institut de Chimie Moléculaire de Grenoble, FR CNRS
2607, Université Joseph Fourier Grenoble
I, BP 53, 38041 Grenoble Cedex
9, France
bDepartment of
Biotechnology Engineering, Ben-Gurion University of the Negev, P.O. Box 653,
84105 Beer-Sheva Israel
cIlse Katz Center for Meso- and Nano-scale Science and
Technology, Ben-Gurion University, P.O. Box 653, 84105 Beer-Sheva,
Israel
dThe National Institute
for Biotechnology in the Negev, P.O. Box 653, 84105 Beer-Sheva,
Israel
Available online 10 July 2006.
Abstract
A
polymeric film consisting of two distinct monomers such as pyrrole–benzophenone and tris(bipyridine pyrrole) ruthenium
(II) units was electrogenerated and used for the
photochemical grafting of T7 bacteriophages
displaying a specific West Nile virus (WNV) epitope.
The resulting electrodes were applied to the amperometric
detection of WNV antibody at 0 V in aqueous electrolyte via secondary peroxidase-labeled antibody, hydrogen peroxide and
hydroquinone as the enzyme substrate. A calibration curve based on different
WNV–antibody dilutions ranging from 10 to 106 was created with a
relatively short response time of 30 s after substrate injection.
Keywords:
Article
Outline
1. Introduction
2.1. Chemicals
2.2. Cloning of
p15x2 in T7 phage
2.3. Amplification
and purification of p15x2-T7 phages
2.4. Apparatus
2.5. Modification
of the electrodes
2.6. Immobilization
of bacteriophages
2.7. WNV-immunosensor preparation
3. Results
3.1. Electrochemical
polymerization and characterization of copolymer films poly(1–2)
3.2. Permeability
of various polymeric films
3.3. Development
of the immunosensor configuration and detection of
the WNV antibody
4. Conclusions
WNV infection has typical clinical symptoms
and positive results can be obtained with specific laboratory tests [6]. Serum or
cerebrospinal fluids are either tested for the presence of IgM
and IgG antibodies by ELISA immunoassays or for viral
RNA detection with RT-PCR in reference laboratories. Today, there are six major
types of assays for the detection of WNV antibodies such as complement
fixation, hemagglutination, the plaque reduction
neutralization test, immuno-fluorescence assays,
enzyme-linked immunosorbent assays (ELISA) and microsphere immunoassays [8]. More
progress has been recently reported by creating a sensitive ELISA–optical fiber
methodology using immobilization of inactivated virions
via argon-phase silanization which provided a lower
detection limit of WNV antibodies such as an analyte
dilution by 106 [9].
With the aim
to develop a portable and inexpensive immunosensor
for the determination of WNV antibody, our work has also focused on an amperometric transduction of the immunoreaction
with the WNV phage-displayed epitope immobilized on
an electrode surface. Recently, biomolecule
immobilization onto electropolymerized photoactivable films was reported, combining elegantly the
advantages of the photografting process to those of
the electrochemical addressing [10]. In particular, the immobilization of biomolecules by light is topically addressable and easily
applicable to a wide variety of biomolecules. The electropolymerization of a pyrrole–benzophenone derivative provided thus the first example of
a polymer film allowing upon irradiation the reagentless
covalent grafting of proteins and enzymes [10], [11] and [12]. The generation of the benzophenone
excited triplet state, requires a low activation energy and is chemically stable.
Electropolymerized poly(pyrrole–benzophenone) films provide similar photografting
properties as benzophenone solutions do, leading to
the anchoring of a protein monolayer.
However, the high hydrophobic character of
these polymers leads in aqueous media to non-permeable films. This constitutes
a major drawback for the development of immunosensors
based on an amperometric transduction. The latter
requires indeed the permeation of electroactive
compounds through the polymer to reach the underlying electrode surface where
an oxidation or reduction process must take place.
Taking this into consideration, we report
here the design of copolymeric films electrogenerated from two pyrrolic
monomers: a photoactivable pyrrole–benzophenone (monomer 1) and a
cationic tris (bipyridine–pyrrole) ruthenium complex (monomer 2)
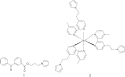
(Fig. 1).
Fig. 1. Structure of monomers 1 and 2.
Thanks to the presence of three pyrrole groups, monomer 2 can
confer, beside a polycationic character, a rigid
cross-linked structure to the resulting copolymer. In contrast to the linear
skeleton of the poly(pyrrole–benzophenone)
films reported until now, this effect may be exploited to imprint an expanded
structure to the polymeric film for facilitating the permeation of redox species.
The immobilization of a WNV phage-displayed epitope onto photoactivable
copolymers able to generate under irradiation several covalent bindings between
polymerized benzophenone groups and a phage entity
was then investigated. In addition, the potentialities of the resulting phage
electrode were examined for the amperometric immunosensing of WNV antibody.
2.1. Chemicals
Bovine serum albumin (BSA, A-3803), and polyoxyethylenesorbitan monolaurate
(Tween 20, P7949) were purchased from Sigma while
goat anti-human IgG H&L HRP are from OEM-Concepts
(G5-G10-2).
IgG preparations from pooled Israeli donors
(IVIG-IL; Omr-IgG-am 5% intravenous IgG, lot E09071) and American donors (IVIG-US, lot G09402)
containing 50 mg/ml IgG were a gift from Pr. Orgad Laub of Omrix
Biopharmaceuticals (Ness-Ziona, Israel). IVIG-IL
already showed prophylactic and therapeutic efficacy in treating WNV infection
in mice [13] and was successfully employed in clinical
treatment of patients with immunosuppression who had
WN fever [14] and [15].
Monomers 1 and 2
were synthesized as described in [10] and [16], respectively. All other chemical reagents
were purchased from Sigma–Aldrich. Water was doubly distilled using a quartz
apparatus.
2.2. Cloning of p15x2 in T7 phage
The sequence of the WNV epitope
Ep15 consists in a sequence of 15 amino acids from the domain III of the WNV envelope
protein. A double-strand DNA corresponding to the peptide GGG-p15-GGG-p15
(Ep15x2) was synthesized (
2.3. Amplification and purification of p15x2-T7
phages
A volume of 50 ml LB-kanamycin was inoculated with a single colony of OrigamiB and incubated overnight at 37 °C. Then,
5 ml was introduced in 500 ml fresh LB-kanamycin.
At OD600 = 1, the bacteria were infected with
0.001 MOI of phage. The solution was incubated in a rotary shaker at
37 °C until cell lysis was observed. The lysate was then clarified by centrifugation at
8000 × g during
10 min and the supernatant containing the Ep15x2-T7 phages was mixed with
50 g PEG-8000. The mixture was gently mixed overnight at 4 °C and
then centrifuged at 8000 rpm for 10 min at 4 °C. The supernatant
was decanted and the PEG pellet resuspended in
1.5 ml of 1 M NaCl, 10 mM Tris–HCl
pH8 and 1 mM EDTA. The suspension was finally
centrifuged 10 min at 10,000 rpm to remove the PEG and the
supernatant transferred in a sterile 1.5 ml tube for further use.
2.4. Apparatus
Cyclic voltammetry
studies were undertaken in a standard three-electrode cell using an EG&G
173 potentiostat/galvanostat and an EG&G Parc Model 175 Universal Programmer (from Princeton Applied
Research,
2.5. Modification of the electrodes
The electropolymerization
of monomers or monomer mixtures (2 mM) was
carried out in organic solution (CH3CN + 0.1 M TBAP)
by controlled potential electrolysis at +0.85 V versus Ag | Ag+
reference electrode. The polymerization process was stopped when an anodic
charge density of 2.5 mC cm−2
was reached. The resulting modified electrodes were characterized in CH3CN + 0.1 M
TBAP free of monomer and then transferred in CH3CN containing
0.1 M LiClO4. Cation exchange was
performed by cycling the resulting modified electrode between −1.0 and
+1.3 V. The upper limit of the potential scan region was set at
+1.3 V to over-oxidize the polypyrrole skeleton
and hence to suppress the electrochemical signal of the polypyrrole
electroactivity.
2.6. Immobilization of bacteriophages
A phage aqueous solution (20 μL) was deposited on the electrode modified by copolymeric films and left to evaporate. The resulting
electrodes were then irradiated under Ar atmosphere
at λ = 355 nm
for 5 min. The irradiation of modified electrodes covered by adsorbed
phages was performed through an optical fiber using a mercury lamp (medium
pressure, 500 W) with a 68910 Arc lamp power supply, both from Oriel
Instruments. Filters from Oriel instruments are used to select the wavelength
at 355 nm and to block IR rays, preventing overheating of the optical
fiber and electrode surface.
2.7. WNV-immunosensor
preparation
The polymer-phage-modified glassy carbon
electrodes were then used in the creation of a series of WNV-immunosensors. To fabricate highly specific immunosensors, a blocking step using phosphate-buffered
saline (0.1 M PBS, pH 7.2) containing 5% (w/v) bovine serum albumin (BSA)
was performed. This blocking solution was prepared daily. The WNV–antibody was
diluted with 1% (w/v) BSA/PBST (PBS containing 0.05% (v/v) Tween-20), which was
kept at 4 °C for 1 week.
The concrete steps for a WNV-immunosensor fabrication are: deposition of 20 μl of the blocking solution onto the surface of a
polymer-phage modified glassy carbon electrode which is left to react for
1 h at room temperature. Then, the electrodes are rinsed with PBS (pH 7.2)
for 5 min before adding 20 μl of several analyte (anti-WNV-IgG) dilution
ranging from 10 to 106. The modified glassy carbon electrodes were
then rinsed and washed once with PBST for 10 min and then thrice for
3 min each. Subsequently, the electrodes were incubated with 20 μl of a solution containing the goat anti-mouse IgG peroxidase-labeled antibodies
(102) for 20 min and then rinsed and washed once with PBST for
10 min and then thrice for 3 min before performing the amperometric measurements.
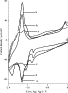
3.1. Electrochemical polymerization and
characterization of copolymer films poly(1–2)
After transfer in a monomer-free electrolyte
solution, electrodes modified by a pure poly2 film
exhibited three successive reversible couples corresponding to the one-electron
reduction of each bipyridine ligand
at −1.68, −1.90, and −2.20 V, respectively. It was previously demonstrated
that poly1 films presented in the negative
region, only one redox system at −1.85 V, attributed
to the one-electron reduction of the polymerized benzophenone
groups [10]. Poly(1–2)
films were electrogenerated from acetonitrile
solutions containing various mixtures of monomers (2 mM),
namely 1–1, 3–1 and 6–1 ratios of monomers 1 and 2
(1/2). Fig. 2 presents
the electrochemical characteristics of the three poly(1–2)
films, in CH3CN + 0.1 M TBAP. For an equimolar mixture of both monomers, redox
responses of the benzophenone groups and bipyridine ligands overlay in the
negative potential region. As expected, the current intensity of the redox system at −1.83 V, ascribed to the polymerized benzophenone, increases with the increase in monomer 1
proportion while the three successive reversible peak systems due to the
ruthenium complex tend to disappear. The polymerization process was carried out
in the presence of a bulky electrolyte cation (TBA+)
to create an expanded polymeric structure. The modified electrodes were then
transferred into CH3CN + 0.1 M LiClO4, to
exchange the incorporated TBA+ by Li+ cations.
In addition, the potential applied to these electrodes was repeatedly cycled
between −1.0 and +1.3 V. This induces, via the Ru
(II/III) transition, an electrolyte permeation through the coating to maintain
its electro-neutrality. As a consequence, the bulky and hydrophobic TBA+
were replaced by the smaller and hydrophilic Li+ cations
within the films. Since the Ru complex contains 3D
pre-oriented pyrrole groups through its three bipyridine ligands, its presence
in the copolymers led to a cross-linked structure that should retain the
imprinting effect of TBA+ [17]. The
replacement of TBA+ by smaller Li+ thus may provide cavities
within the cross-linked polymeric structure that would enhance the permeability
of the polypyrrole films.
Fig. 2. Cyclic voltammograms of electrodes modified with poly(1, 2) films at the ratios 6–1 (a), 3–1 (b), and 1–1 (c) in CH3CN + 0.1 M TBAP, v = 100 mV s−1.
3.2. Permeability of various polymeric films
In order to
compare the permeability of the different polymeric films, the permeation of a redox mediator (ferrocene (Fc)) and its water-soluble derivative (Fc(COOH)2)
at poly(1–2)
electrodes was investigated by cyclic voltammetry in
both organic and aqueous media. Fig. 3 shows the cyclic
voltammograms of Fc
(2 mM) in CH3CN + 0.1 M
LiClO4 recorded at poly(1–2)
electrodes elaborated with 1–2
ratios of 1–1, 3–1 and 6–1. No appreciable change in current intensity of the
peak system of ferrocene (2 mM)
appeared between copolymers based on ratios 3–1 and 6–1 while a slightly more
intense and reversible redox couple was observed for
the equimolar ratio. This reflects a better
permeability that may be attributed to a more efficient imprinted effect with
increasing 2 proportion in the copolymer.
Nevertheless, these similar permeabilities towards Fc were ascribed to a polymer swelling in organic solution.
A similar experiment carried out with Fc(COOH)2
(2 mM in a 0.1 M LiClO4 aqueous
solution) as the electroactive probe showed that the Fc(COOH)2 oxidation at the underlying glassy
carbon electrode was completely suppressed by the presence of copolymers
generated with an excess of 1. It appears
that the high proportion of 1 in the poly(1–2)
films prevented the formation of a rigid polymeric network leading thus to a
polymer collapse in water that blocks the Fc(COOH)2
permeation. In contrast, the cyclic voltammogram
recorded at the electrode covered by the equimolar
copolymer clearly exhibits the reversible one-electron oxidation of the redox mediator at E1/2 = 0.49 V
followed by a reversible peak system at E1/2 = 0.95 V
due to the one-electron oxidation of the polymerized ruthenium complex (Fig. 4). This
unambiguously indicates the beneficial effect on the film permeability brought
by the electrolyte imprint. Consequently, only the photografting
properties of poly(1–2)
films made from an equimolar mixture of monomers were
examined.
Fig. 3. Cyclic voltammograms of Fc (2 mM) at electrodes modified by poly(1, 2) films (ratios 6–1 (a), 3–1 (b), and 1–1 (c)) in CH3CN + 0.1 M LiCLO4, v = 100 mV s−1.
Fig. 4. Cyclic voltammograms of Fc(COOH)2 (2 mM) at electrodes modified by poly(1–2) films, ratios 1–1 (a) and 6–1 (b), in 0.1 M LiCLO4 aqueous solution, v = 100 mV s−1.
3.3. Development of the immunosensor
configuration and detection of the WNV antibody
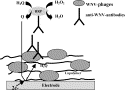
After the
adsorption of phages onto poly(1–2)
films, the latter were covalently linked through the light activation of the
polymerized benzophenone groups at λ = 355nm. In order to
investigate the result of the photochemical reaction, the modified electrodes
were incubated with different samples of the WNV antibody, the dilution ranging
from 10 to 106. Then, the resulting electrodes were incubated with a
secondary antibody labeled with horseradish peroxidase
acting as the marker for the WNV antibody. The recognition of the WNV antibody
bound to the phage, conferred thus a peroxidase
activity to the modified electrode. Subsequently, all the WNV-immunosensors were soaked into 0.1 M PBS (20 ml,
pH 7.2) containing a peroxidase substrate:
hydroquinone (2 mM) and potentiostated
at 0 V. After the injection of hydrogen peroxide (2 mM), the amperometric signal
corresponding to the reduction of the enzymatically
generated quinone was recorded as a function of the
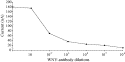
WNV antibody dilution (Fig. 5). It
appears that stable responses appeared with a fast response time (30 s)
illustrating the copolymer permeability. The resulting maximum current values
were then used for elaborating a calibration curve for the WNV antibody (Fig. 6). Each
point from the calibration curve represents an average of three different immunosensor responses. A current decrease was observed for
antibody titers ranging from
Fig. 5. Functioning principle of the amperometric WNV-immunosensor.
Fig. 6. Calibration curve for WNV antibody obtained at poly(1–2) films (ratio 1–1) via an electro-enzymatic immunoassay. Applied potential 0 V in 0.1 M PBS (20 ml, pH 7.2).
We have
demonstrated herein the potentialities of an electrogenerated
polypyrrole film composed of benzophenone
and tris(bipyridine pyrrole) ruthenium (II) groups, for the efficient
photochemical immobilization of large biological entities with retention of
their molecular recognition properties. Moreover, the imprinted permeability of
the copolymer due to the Ru complex as cross-linking
agent, allowed the use of amperometric transduction
for such biodevices.
EU is
thanked for funding under the 6th Framework contract: NMP-A-CT-2003-505485-1. Gregoire Herzog is grateful to the CNRS for postdoctoral
fellowship. The sequence of the putative WNV epitope
was generously provided by the Pr. Bracha Rager-Zisman (Department of Microbiology and Immunology,
[1] B. Nosal and R. Pellizzari,
[2] Health
[3] A.A. Leis,
J. Fratkin, S.S. Dobrivoje,
T. Harrington, R.M. Webb and S.A. Slavinski, West
Nile poliomyelitis, Lancet Infect Dis 3 (2003), pp.
9–10. SummaryPlus | Full Text + Links | PDF (527 K) | View Record
in Scopus | Cited By in
Scopus
[4] J. Li,
J.A. Loeb, M.E. Shy, A.K. Shah, A.C. Tselis and W.J. Kupski et al.,
Asymmetric flaccid paralysis: a neuromuscular presentation of West Nile virus
infection, Ann Neurol
53 (2003), pp. 703–710. Full Text via CrossRef | View Record
in Scopus | Cited By in
Scopus
[5] G.L.
Campbell, A.A. Marfin, R.S. Lanciotti
and D.J. Gubler, West Nile virus, Lancet Infect Dis
2 (2002), pp. 519–529. SummaryPlus | Full Text + Links | PDF (1229
K) | View Record
in Scopus | Cited By in
Scopus
[6] L.R.
Petersen and A.A. Marfin,
[7] B. Murgue, H. Zeller and L.V. Deube,
The ecology and epidemiology of
[8] H.E. Prince
and W.R. Hogrefe, Assays for detecting West Nile
virus antibodies in human serum, plasma, and cerebrospinal fluid, Clin Appl ImmunRev 5 (2005),
pp. 45–63. SummaryPlus | Full Text + Links | PDF (185 K) | View Record
in Scopus | Cited By in
Scopus
[9] S. Herrmann,
B. Leshem, S. Landes, B.R. Zisman and R.S. Marks, Chemiluminescent
optical fiber immunosensor for the detection of
anti-West Nile virus IgG, Talanta 66
(2005), pp. 6–15.
[10] S. Cosnier and A. Senillou, An electrogenerated poly(pyrrole–benzophenone) film for
the photografting of proteins, Chem Commun 3
(2003), pp. 414–415. Full Text via CrossRef | View Record
in Scopus | Cited By in
Scopus
[11] G. Herzog,
K. Gorgy, T. Gulon and S. Cosnier,
Electrogeneration and characterization of photo-activable films and their application for enzyme grafting, Electrochem Commun 7
(2005), pp. 808–814. SummaryPlus | Full Text + Links | PDF (222 K) | View Record
in Scopus | Cited By in
Scopus
[12] T. Konry, A. Novoa, Y. Shemer-Avni, N. Hanuka and S. Cosnier et al.,
Optical Fiber Immuno-sensor based on a poly(pyrrole–benzophenone) film for
the detection of antibodies to viral antigen, Anal Chem 77
(2005), pp. 1771–1779. Full Text via CrossRef | View Record
in Scopus | Cited By in
Scopus
[13] D.
Ben-Nathan, S. Lustig, G. Tam, S. Robinzon,
S. Segal and B. Rager-Zisman, Prophylactic and
therapeutic efficacy of human intravenous immunoglobulin in treating West Nile
virus infection in mice, J Infect Dis 188 (2003), pp.
5–12. Full Text via CrossRef | View Record
in Scopus | Cited By in
Scopus
[14] A. Hamdan, P. Green,
[15] Z. Shimoni, M.J. Niven, S. Pitlick and S. Bulvik, Treatment
of West Nile virus encephalitis with intravenous immunoglobulin, Emerg Infect Dis
7 (2001), pp. 759–769. View Record
in Scopus | Cited By in
Scopus
[16] S. Cosnier, A. Deronzier
and J.-C. Moutet, Oxidative electropolymerization
of polypyridinyl complexes of ruthenium (II)
containing pyrrole groups, J Electroanal Chem
193 (1985), pp. 193–204. Abstract | Abstract + References | PDF (775 K)
[17] S. Cosnier, A. Deronzier
and J.-C. Moutet, Polypyridinyl complexes
of ruthenium (II) having 4,4′-dicarboxyester-2,2′-bipyridine ligands attached covalently to polypyrrole
films. Reinvestigation of the polypyrrole
electrochemical response in poly[tris(N-bipyridylethyl)pyrrole] ruthenium (II) films, J Electroanal Chem
285 (1990), pp. 133–147. Abstract | Abstract + References | PDF (809 K) | View Record
in Scopus | Cited By in
Scopus
![]() Corresponding author. Tel.: +33
4 76 51 49 98; fax: +33 4 76 51 42 67.
Corresponding author. Tel.: +33
4 76 51 49 98; fax: +33 4 76 51 42 67.
|
Enzyme and Microbial Technology |
|
|
|
|
|